

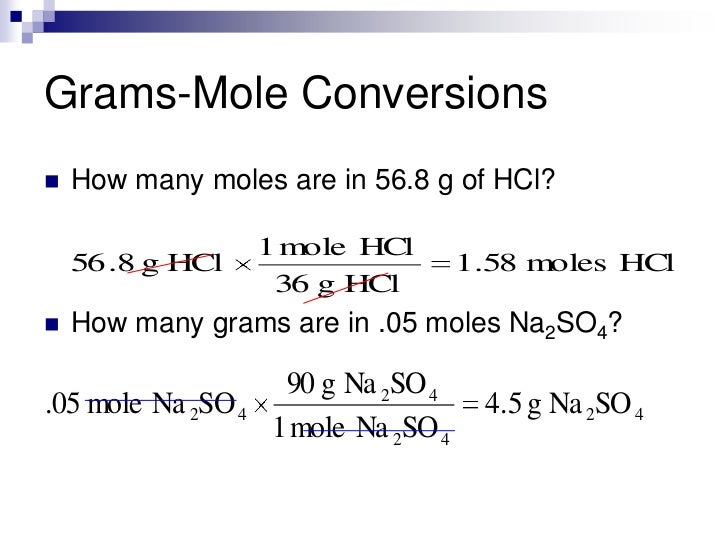
Now, for Chemistry when dealing with atoms, compounds or particles of elements, the 'convenient number' is the mole, which makes the use of the atomic mass numbers on the Periodic table manageable and much easier to use both in theoretical calculations and practical experimentation with chemicals. The last item, the salt, it would not be feasible to count out grains or even to determine the grain size being consistent, but would be more like the problems encountered for the much smaller atom/compound/molecule. I would just like to add maybe an even more basic explanation that I would employ to start with: when going to the grocery store to shop for you and your family, you would not pick up eggs individually anymore, or say 27 mL of milk or a specific amount of grains of salt (say 5000 grains), you would get say a donzen eggs, a litre/gallon of milk and specific mass/weight in gram/ounce of salt depending upon which unit of measure you use, this being more convenient and manageable number(s) when shopping.

I agree completely with Just Keith's answer of the over complication of this video along with his great detail on the background and mathematics of the mole and atomic mass unit. I was just trying to show why 1 mole of amus equals 1g. Generally when working with Avogadro's number and the mass of a proton or neutron, you wouldn't substitute the values in. I suggest comparing line-by-line with the above example to see the similarities.Įxample 1: Let's determine the mass of 1 dozen eggs.Įxample 2: Show that 1 mole of 12C is 12g. I've included an example below showing the same concept to the above example but in a more familiar context. I also think they chose to invent the mole because the mass of a proton or neutron is such a small number that they needed a way to descibe the mass in quantities that we can work with. I think that 1 mole was chosen to be 6.02x10^23 so that when it was multiplied by the amu it would give a nice number like 1g (shown above). (Please note: Some of the numbers above have been approximated and rounded but for simplicity I've left that stuff out.)
#MOLES TO GRAMS FULL#
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types.Hi, this is the way I came to understand the concept of a mole. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.Ĭonversion calculator for all types of measurement units. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance.
#MOLES TO GRAMS HOW TO#
This site explains how to find molar mass.įinding molar mass starts with units of grams per mole (g/mol). The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert. These relative weights computed from the chemical equation are sometimes called equation weights.Ī common request on this site is to convert grams to moles. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Grams KOH to moles, or enter other units to convert below: Enter two units to convert From: You can do the reverse unit conversion from


 0 kommentar(er)
0 kommentar(er)
